“Before Four Weeks: Updated Vaccination Recommendations for Neonatal Puppies and Kittens”, MMPC Learniverse webinar
“New vaccination recommendations for neonatal puppies and kittens”, Animal Care Expo 2025 presentation slides
Use of the modified live canine distemper virus and parvovirus vaccines in neonatal puppies and kittens in animal shelters and other environments with meaningful risk of exposure.
Recommendation: When meaningful risk is present, both vaccination at the earliest possible time and minimizing exposure should be used in combination to the greatest extent possible.
When exposure to panleukopenia, parvo, or distemper is a meaningful risk in shelters, immediate vaccination of puppies and kittens with DAPP and FVRCP respectively, beginning at the time of birth, intake, or prior to intake is a potentially life-saving practice that poses only very rare and largely theoretical risks. Neonatal vaccination may also be indicated for puppies and kittens in any environment where access to care is limited and exposure risk is significant. While early vaccination may not effectively immunize all pups or kittens, for neonates with low levels of maternal antibodies, the chance to start making antibodies earlier could save them from severe disease and likely death from CDV, FPV, and CPV-2 should they be exposed.
Recommended vaccination schedules outside of the shelter context have been based on the assumption that maternal antibody will both protect neonates and prevent vaccine efficacy. However, these assumptions do not necessarily apply to animals presented to shelters or living in communities where access to care is limited. Studies of animals presented to animal welfare organizations have found immunity in as few as one third of cats and dogs in communities studied. Intact animals presented to shelters are more likely to lack immunity and by definition are the source of puppies and kittens. One study of dogs found nearly two thirds of dogs entering a Florida shelter had very low to no protective antibodies to distemper, parvo, or both, with intact dogs at higher risk than those who had been neutered or spayed[1]. In a study of cats presented to animal shelters, also in Florida, 60% of cats had insufficient antibody levels for protection (<1:40) against FPV. Intact cats were also at higher risk than those who had been neutered or spayed [2]. A study of trapped cats from the community, presented for sterilization, found that 67% of free roaming cats did not have sufficient antibody levels to be protective against FPV [3].
These studies suggest that in many communities, kittens and puppies presented to a shelter are unlikely to have reliably acquired maternal immunity. Even when antibodies are theoretically present in the mother due to prior vaccination or exposure to field strain virus, transfer of antibodies to puppies and kittens may be insufficient e.g. due to poor maternal nutrition or inadequate nursing.
Reflecting this understanding, recommendations for DAPP vaccination for puppies and FVRCP for kittens in shelter settings have evolved to begin at four weeks of age with revaccination every 2 weeks [4]. Although many vaccines are only labeled for use in cats and dogs 6 weeks and over, recommendations for this off-label use of the vaccine products reflect the benefit of potentially providing immunity to fully virulent field strain virus which far outweighs any risk from current MLV vaccines.
The recommendations to start vaccination at 4 weeks of age rather than even younger have been paired with recommendations to keep animals below that age out of shelters. This historical age cut off must be reconsidered in light of the reality that foster care safety nets do not yet always match the needs for placement, leaving many shelters to house neonatal animals in environments with meaningful risk. In addition, many puppies and kittens are born and raised in homes and community environments where there is a need to protect them from risk. Although the immune system is not fully matured, it is known that vaccination can be safe and effective in newborns of many species [5].
Research demonstrates that danger from vaccination, even in neonatal puppies, is uncommon and minimal, especially when compared to disease resulting from exposure to field strains. In a study by Gerber et al. in 1976, no adverse responses were noted after pups were vaccinated at two weeks of age with canine distemper vaccine (CDV) and human measles virus vaccine (HMV)[6]. Chappuis (1998) studied the ability of neonatal puppies to respond to vaccination and the author was able to safely vaccinate groups of one day old puppies with MLV CPV-2 vaccines. None of the puppies had adverse reactions to the vaccines, and many were able to mount an immune response. Immunized pups maintained antibody levels steadily over the course of 90 days while the unvaccinated control puppies had maternal antibodies that waned steadily over time[7].
There are no published reports of modern vaccines inducing disease in kittens. One study, that has been cited to support delaying vaccination of kittens for FPV until after four weeks of age, is actually a case report describing a pregnant stray cat with unknown history who was vaccinated with a MLV panleukopenia vaccine [8]. The kittens in the report were never vaccinated. Two kittens had cerebellar hypoplasia. The authors noted that the infection was likely due to exposure of the mother to field strain during the prenatal period rather than from exposure to vaccine virus.
The most substantial risk related to vaccination in neonates would come from assuming that all animals are fully immunized following vaccination while minimizing the importance of protecting them from environmental risk. While some neonates will be effectively immunized, some proportion may fail to mount a protective response due to maternal antibody or inability of the immature immune system to fully respond. The likelihood of responding immunologically improves with age (and declining maternal antibody levels) and, because neonates cannot completely thermoregulate on their own, may be improved by ensuring neonatal puppies and kittens are kept adequately warm.
When susceptible animals and pathogens are present in the environment both vaccination at the earliest possible time and minimizing exposure should be used in combination to the greatest extent possible. This is similar to recommendations, and the supporting rationale, already commonly accepted for pregnant dogs and cats entering shelters [4]. Minimizing the time that puppies, kittens, and pregnant animals are in the shelter environment also reduces the risk from the myriad other infectious threats and risks to well-being for which no vaccine is available.
The increasingly severe access to care crisis in veterinary medicine leaves a growing number of owners unable to get their dogs and cats vaccinated or spayed/neutered. As a result, the infectious disease risk in areas where access to care is limited may more closely resemble the risk seen in shelters than the scenario envisioned by traditional vaccine recommendations. Therefore, these recommendations for early vaccination are also applicable and important for puppies and kittens outside of shelters, in any context where environmental exposure poses a meaningful risk. Vaccination of neonatal puppies and kittens is also indicated when that may be the only time the animals are seen, such as at a temporary clinic.
Sandra Newbury, DVM, Dip. ABVP (Shelter Medicine)
Director – UW Shelter Medicine
Associate Professor
Department of Medical Sciences
University of Wisconsin – Madison
School of Veterinary Medicine
www.UWsheltermedicine.com
Kate F. Hurley, DVM, MPVM, Dip. ABVP (Shelter Medicine)
Director, Koret Shelter Medicine Program
Center for Companion Animal Health
UC Davis School of Veterinary Medicine
www.sheltermedicine.com
Acknowledgements: Thanks to Carrie Allen, DVM, Maddie’s Shelter Medicine Research Fellow, UW Shelter Medicine, Univeristy of Wisconsin-Madison School of Veterinary Medicine for her substantial contribution to the research and writing of this document and to Aleisha Swartz, DVM for her thoughtful community engaged review.
Appendix follows below
[1] Lechner ES, Crawford PC, Levy JK, Edinboro CH, Dubovi EJ, Caligiuri R. Prevalence of protective antibody titers for canine distemper virus and canine parvovirus in dogs entering a Florida animal shelter. Journal of the American Veterinary Medical Association 2010;236:1317–21.
[2] DiGangi BA, Levy JK, Griffin B, McGorray SP, Dubovi EJ, Dingman PA, et al. Prevalence of serum antibody titers against feline panleukopenia virus, feline herpesvirus 1, and feline calicivirus in cats entering a Florida animal shelter. Journal of the American Veterinary Medical Association 2012;241:1320–5.
[3] Fischer SM, Quest CM, Dubovi EJ, Davis RD, Tucker SJ, Friary JA, et al. Response of feral cats to vaccination at the time of neutering. J Am Vet Med Assoc 2007;230:52–8. https://doi.org/10.2460/javma.230.1.52.
[4] The Association of Shelter Veterinarians. The Guidelines for Standards of Care in Animal Shelters: Second Edition. Journal of Shelter Medicine and Community Animal Health 2022;1:1–76. https://doi.org/10.56771/ASVguidelines.2022.
[5] Demirjian A, Levy O. Safety and Efficacy of Neonatal Vaccination. Eur J Immunol 2009;39:36–46. https://doi.org/10.1002/eji.200838620.
[6] Gerber JD, Marron AE. Cell-mediated immunity and age at vaccination associated with measles inoculation and protection of dogs against canine distemper. Am J Vet Res 1976;37:133–8.
[7] Chappuis G. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine 1998;16:1468–72. https://doi.org/10.1016/S0264-410X(98)00110-8.
[8] Sharp NJH, Davis BJ, Guy JS, Cullen JM, Steingold SF, Kornegay JN. Hydranencephaly and Cerebellar Hypoplasia in Two Kittens Attributed to Intrauterine Parvovirus Infection. Journal of Comparative Pathology 1999;121:39–53. https://doi.org/10.1053/jcpa.1998.0298.
Appendix for:
Use of the modified live canine distemper virus and parvovirus vaccines in neonatal puppies and kittens in animal shelters and other environments with meaningful risk of exposure.
Newbury and Hurley 2024
1 Lechner ES, Crawford PC, Levy JK, Edinboro CH, Dubovi EJ, Caligiuri R. Prevalence of protective antibody titers for canine distemper virus and canine parvovirus in dogs entering a Florida animal shelter. Journal of the American Veterinary Medical Association 2010;236:1317–21.
Key points:
- The majority of dogs (65%) admitted lacked immunity to either CDV or CPV, or both CDV and CPV.
- Intact dogs were much less likely to have antibody levels sufficient to protect from CDV and CPV than dogs who have been spayed / neutered.
In this paper, dogs surrendered by owners or admitted as strays at a large municipal shelter in Florida were evaluated for the presence of a protective antibody titer (PAT) for both CDV and CPV. A total of 441 dogs had blood samples taken for antibody titer assay. The authors found that 70% of neutered / spayed dogs had antibodies indicating protection, while only 30% of intact dogs had antibodies indicating protection. “The proportion of neutered dogs with PATs for both viruses (44/63 [69.8%]) was significantly (P < 0.01) higher than the proportion of sexually intact dogs with PATs for both viruses (109/368 [29.6%]).”
Below are the numbers presented:
63 neutered dogs 44 protected 30% unprotected
368 intact dogs 109 protected 70% unprotected
431 total dogs 153 protected
Only a little more than one third of all dogs are protected (35%), while nearly two thirds (65%) of all dogs were unprotected. Younger dogs were also more likely to lack immunity than older dogs with 83% of those less than one year lacking immunity. Of interest, for dogs with immunity to only one of the pathogens, immunity to only CPV was more common indicating that CPV is more frequently encountered and survived as a natural infection.
2 DiGangi BA, Levy JK, Griffin B, McGorray SP, Dubovi EJ, Dingman PA, et al. Prevalence of serum antibody titers against feline panleukopenia virus, feline herpesvirus 1, and feline calicivirus in cats entering a Florida animal shelter. J Am Vet Med Assoc 2012;241:1320–5.
Key points:
- The majority (60%) of cats admitted did not have sufficient antibody to be protective for FPV.
- Intact cats were over twice as likely to lack immunity compared to spayed / neutered cats. (66% vs 30%)
Cats relinquished to an open admission municipal shelter in Florida were evaluated for the presence of antibodies against FPV, FHV-1, and FCV 24 hours after intake.
Results included: “Of 347 cats, 138 (39.8%), 38 (11.0%), and 127 (36.6%) had antibody titers ≥ 40, ≥ 8, and ≥ 32 (ie, seropositive) against FPV, FHV1, and FCV, respectively. Factors associated with seropositivity included being neutered, age ≥ 6 months, and being relinquished by an owner.”
“On multivariable analysis to identify factors significantly associated with cats being seropositive for FPV antibodies, evidence suggested that source, environment of origin, signs of previous caregiving, and vaccination < 24 hours prior to blood sample collection were each significantly associated (ie, collinear) with neuter status; therefore, only one of the collinear terms could be included in the model. The inclusion of neuter status provided the best model fit. Thus, the final multivariable model for FPV seropositivity included only neuter status; neutered cats were significantly (P < 0.01) more likely to be seropositive for FPV antibodies than were sexually intact cats (OR, 4.4; 95% CI, 2.4 to 8.3).”
Numbers presented for FPV:
Total all cats 347 139 Protected (60.2%) 208 unprotected (39.8%)
Intact 291 99 protected (34%) 192 unprotected (66%)
Neutered 56 39 protected (70%) 17 unprotected (30%)
3 Fischer SM, Quest CM, Dubovi EJ, Davis RD, Tucker SJ, Friary JA, et al. Response of feral cats to vaccination at the time of neutering. J Am Vet Med Assoc 2007;230:52–8. https://doi.org/10.2460/javma.230.1.52.
Key points:
- “Feral” cats presented for TNR clinic had similarly low levels of immunity to FPV as intact cats in the previous study (DiGangi 2012).
- 67% of cats (41/61) had antibody level below a range that would be considered protective
- Because only free roaming unsocialized, unsterilzed cats were presented, the other 33% were almost certainly positive from exposure to field strain virus rather than vaccination.
Sixty-one “feral” cats were evaluated for antibody titers for FPV, FHV-1, FCV, and RV by drawing blood at the time of sterilization. “Among the 61 cats, 28 (46%) had serum antibodies against FPV (median titer, 0; IQ range, 0 to 800) at the time of surgery, indicating previous exposure or vaccination, but only 20 (33%) had titers in the protective range.”
Of interest, data from this study suggests some cats are likely experiencing exposure and survival to parvoviruses in the field. That information parallels information from Lechner 2010 study in dogs described above.
4 The Association of Shelter Veterinarians. The Guidelines for Standards of Care in Animal Shelters: Second Edition. Journal of Shelter Medicine and Community Animal Health 2022;1:1–76. https://doi.org/10.56771/ASVguidelines.2022.
Key points:
- Vaccination should start at an early age.
- The benefits of vaccination for pregnant animals outweigh the risks of not vaccinating.
- References cited in support of a theoretical risk of cerebellar hypoplasia as a complication of MLV panleukopenia vaccination of pregnant cats do NOT lead back to any studies or reports involving neonatal vaccination nor conclusive evidence of harm even to pregnant cats or their offspring.
“Vaccination schedule for animals housed in shelter facilities: MLV DAPP; SQ; Dog; Starting age 4 weeks; intake, every 2 weeks” and “MLV FVRCP; SQ; Cat; Starting age 4 weeks; intake, every 2 weeks.”
The Guidelines also includes recommendations for vaccination of pregnant animals at time of intake. “Animals housed in shelters should be vaccinated with core vaccines even if ill or pregnant, as the individual and population risks of not vaccinating outweigh the small risk of vaccination.”
This Second Edition, Section 6.4.2 also includes the statement below:
“Cerebellar hypoplasia is a theoretical complication of MLV panleukopenia vaccination of pregnant cats; however, the risk of abortion, maternal, and kitten death due to panleukopenia generally outweighs this concern in shelters.”
While this statement about cerebellar hypoplasia describes the risk as theoretical and goes on to support vaccination of pregnant cats, a citation is made that references it to a complex chain of articles. The statement cited in first article in this chain, Barrs 2019 (ref number 37 in the Guidelines 2nd ed. See below for Additional Reference A) is “Administration of MLV vaccines against FPV in kittens younger than 4 weeks of age is not recommended because of the risk of cerebellar hypoplasia.”
This statement in Barrs 2019 cites the AAFP 2013 Vaccine Guidelines (Additional reference B) as a supporting reference. The AAFP 2013 Vaccine Guidelines in turn have a similar statement. Sharp 1999(8) is cited as a supporting reference to delay vaccination.
Sharp 1999 is cited in this new recommendation document and is more fully described in this Appendix below(8). Sharp 1999 discusses only one case, a pregnant queen with an unknown history, who was vaccinated. The paper does not report disease resulting from vaccination of neonatal animals at all.
None of the articles in this chain of supporting references describe cerebellar hypoplasia, maternal, or kitten morbidity and mortality that clearly resulted from early age vaccination (and not natural infection) of neonatal kittens or vaccination of pregnant queens leaving those statements unsupported. (See Sharp et al below)
5 Demirjian A, Levy O. Safety and Efficacy of Neonatal Vaccination. Eur J Immunol 2009;39:36–46. https://doi.org/10.1002/eji.200838620.
Key Points:
- Several vaccines are successfully given to human babies in the neonatal period without problems.
- Also noted is the importance of providing vaccines at a point of contact when the patient is accessible.
Statements cited:
“birth is a major point of healthcare contact globally meaning that effective neonatal vaccines achieve high population penetration.”
Below are some of the specific examples given in the article:
“Hepatitis B vaccine is recommended for administration at birth in the US, though the oral polio and BCG (Bacillus Calmette-Guerin) vaccines are also given in other parts of the world.”
“Bacillus Calmette-Guerin, a live vaccine against tuberculosis, demonstrates that a single dose of vaccine administered at birth can in principle confer lifelong protection.”
“Hep B vaccine confers strong protection, significantly reducing infection rates [9], and has also been shown to have therapeutic value when administered to Hep B-infected infants in conjunction with Hep B immunoglobulin.”
6 Gerber JD, Marron AE. Cell-mediated immunity and age at vaccination associated with measles inoculation and protection of dogs against canine distemper. Am J Vet Res 1976;37:133–8.
Key points:
- Vaccination at about two weeks of age was not harmful to pups.
- Pups in this study were not SPF and had varying low levels of detectable antibody (most likely maternally derived) before vaccination. While immunity did not develop as a result of vaccination this study suggests that pups with MDA would not be harmed by neonatal vaccination.
“5 pups were vaccinated at between 16 and 30 days of age with canine distemper vaccine, and 5 pups of the same age were vaccinated with measles vaccine.” Early vaccination was not harmful to either group of pups and they were all monitored out to day 42.
7 Chappuis G. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine 1998;16:1468–72. https://doi.org/10.1016/S0264-410X(98)00110-8.
Key points:
- All domestic species are immunocompetent at birth although additional maturation of the immune response will occur during the neonatal period.
- Pups with no MDA and no detectable antibody were not harmed by vaccination at one day of age.
- Immunity developed as in older pups while no adverse reactions were noted.
- Similar response was noted to Rabies vaccination.
Five SPF puppies were subcutaneously inoculated to CPV at 1 day of age. “No adverse reaction was reported and the post-vaccinal kinetics of antibodies (HAI) between 21 and 91 days post-vaccination was similar to older puppies in terms of level and stability, hence showing the complete maturity of the immune system.”
“These two experiments were designed to be more for academic’ than routine use, but certainly provide evidence of satisfactory immunocompetency of puppies within the first days of life.”
“Vaccination at an early age is advisable because infectious diseases and their consequences are a great risk for young animals. All domestic species are immunocompetent at birth although additional maturation of the immune response will occur during the neonatal period. The main obstacle to successful vaccination in young animals is the presence of blocking levels of maternally-derived antibodies.”
8 Sharp NJH, Davis BJ, Guy JS, Cullen JM, Steingold SF, Kornegay JN. Hydranencephaly and Cerebellar Hypoplasia in Two Kittens Attributed to Intrauterine Parvovirus Infection. Journal of Comparative Pathology 1999;121:39–53. https://doi.org/10.1053/jcpa.1998.0298.
Key points:
- The case of a pregnant queen with an unknown history who was vaccinated when found and adopted is presented.
- Neonatal kittens were not vaccinated as part of this case report.
- A wide range of severe problems were reported in the kittens born from this queen’s pregnancy.
- The study is frequently cited to support recommendations to avoid vaccination in kittens under 4 weeks of age but this report does not describe vaccination of any kitten.
- The authors conclude this study saying “It was not possible to ascertain whether the viral isolate was vaccine-derived or a field virus. There was ample time for her to become infected with field strain FPV either before or even shortly after her vaccination.”
- This case occurred over 25 years ago. No other studies or more definitive case has been reported in the literature.
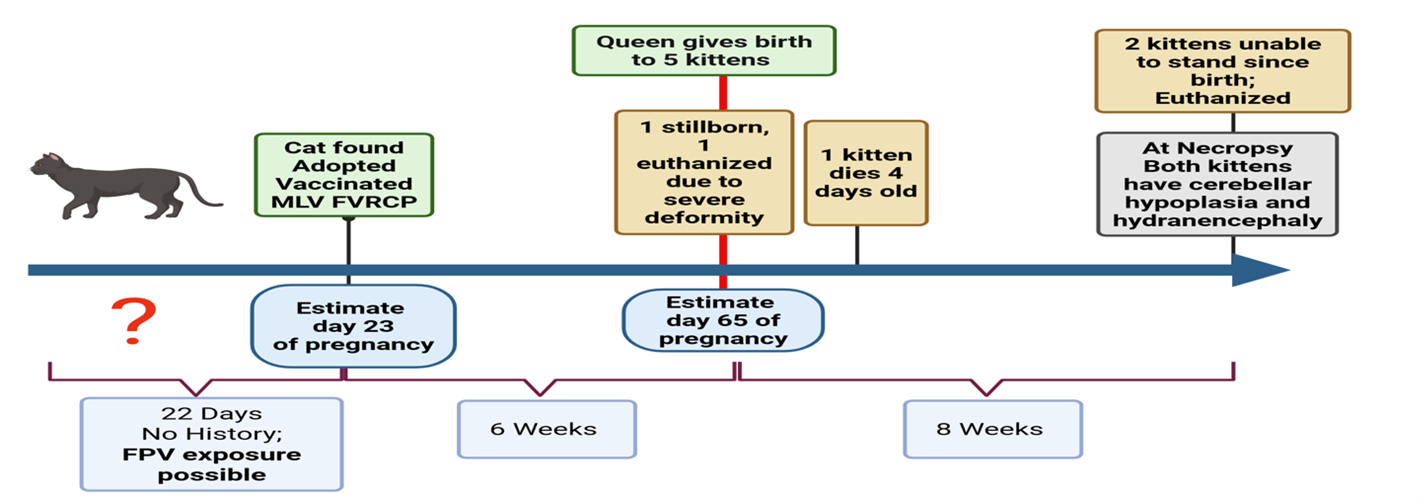
A pregnant female cat aged less than one year was found, adopted, and vaccinated with a live attenuated vaccine containing feline calicivirus, feline rhinotracheitis and panleukopenia viruses on approximately day 20-23 of her pregnancy.
No other history for this female cat was reported. Six weeks after vaccination, the cat gave birth to five kittens. The first was born dead, one was euthanized due to deformities in the pelvic limbs, the next three appeared normal. Of the surviving kittens, one would not suckle and lived for only 4 days. The remaining two kittens (A and B) were presented at 8 weeks of age, with a history of never having been able to stand or walk.
The authors describe brain lesions noted on necropsy of kittens A and B consistent with hydranencephaly and cerebellar hypoplasia. They also describe the use of PCR to identify FPV DNA within the affected brain tissues.
The authors conclude this study saying “It was not possible to ascertain whether the viral isolate was vaccine-derived or a field virus. There was ample time for her to become infected with field strain FPV either before or even shortly after her vaccination.”
“Our findings demonstrate the value of the PCR in confirming the presence of parvoviral DNA in archival brain tissue. The results suggest that in-utero infection with feline parvovirus caused the combined CNS malformations of hydranencephaly and cerebellar hypoplasia”.
Additional References (included in presentation that accompanies this document)
A Barrs VRV. Feline Panleukopenia: A Re-Emergent Disease. Vet Clin North Am Small Anim Pract. 2019;49(4):651–670. doi: 10.1016/j.cvsm.2019.02.006
Key points:
- Barrs 2019 is cited by the Guidelines for Standards of Care in Animal Shelters 2nd to support a statement suggesting risk of cerebellar hypoplasia from vaccination of pregnant queens.
- Barrs 2019 recommends delaying vaccination of very young kittens to after 4 weeks of age because of the risk of cerebellar hypoplasia.
- The statement in Barr is supported by a cited reference(Sharp 1999) that does not report vaccinating neonatal kittens and the authors state that the source of disease reported in kittens born from a queen who had been vaccinated while pregnant may have been field strain virus.
- From Sharp 1999: “It was not possible to ascertain whether the viral isolate was vaccine-derived or a field virus. There was ample time for her to become infected with field strain FPV either before or even shortly after her vaccination.”
The statement from Barrs 2019 is below:
“In shelter environments the core vaccine schedule administration of MLV vaccines against FPVin kittens younger than four weeks of age is not recommended because of the risk of cerebellar hypoplasia.”
The statement above cites the AAFP 2013 guidelines as a supporting reference.
B Scherk MA, Ford RB, Gaskell RM, Hartmann K, Hurley KF, Lappin MR, et al. 2013 AAFP Feline Vaccination Advisory Panel Report. Journal of Feline Medicine and Surgery 2013;15:785–808. https://doi.org/10.1177/1098612X13500429.
The AAFP 2013 Vaccine Guidelines reference Sharp et al 1996 listed above where the authors describe their inability to determine if harm to fetuses was caused by maternal infection with filed strain or vaccine virus. No vaccination of neonatal kittens was described or reported in Sharp et al.1999 (Reference 8 above)
C1 Larson L J., Newbury S, Schultz R D. Canine and Feline Vaccinations and Immunology. In: Miller L and Hurley K, editors. Infectious Disease Management in Animal Shelters. 1st ed., Ames, Iowa: Wiley-Blackwell; 2009. p. 61–82.
C2 Larson Laurie J and Ronald D Schultz. Canine and Feline Vaccinations and Immunology. In: Miller L, Janeczko S, Hurley K, editors. Infectious Disease Management in Animal Shelters. 2nd ed., Newark, New Jersey: John Wiley & Sons, Incorporated; 2021; P191-220
In this chapter (Larson, Newbury, and Schultz 2009) the authors recommend that kittens younger than four weeks of age should not be vaccinated because of severe risk of morbidity and mortality.
“Prior to two weeks of age, the immune system is unlikely to mount an effective immunizing response to many vaccines, and the vaccine virus can cause disease and death at this early age”
This recommendation persists in the second edition (Larson and Schultz 2021). Schultz et al. 1977 is cited in support of this recommendation but describes no detriment from very early age vaccination other than the possibility that the kitten or puppy may not be effectively immunized if younger than 2 weeks of age due to the presence of MDA or hypothermia. (see below Addition Ref D)
D Schultz RD, Appel M, Carmichael L, Farrow B, Canine Vaccines and Immunity Current Veterinary Therapy VI: Small Animal Practice 1977: 1271-1275
Schulz et al. make two statements on neonatal vaccination and vaccination of pregnant animals. The only statement referencing the possibility of harm was in regard to pregnant animals, and acknowledges there is no evidence of harm, just a theoretical concern based on the lack of research, field experience, or knowledge at the time (over 40 years ago). The only statement in regard to vaccination of neonates does not suggest the possibility of harm, only that the response, especially the cell mediated immune response, may be suboptimal due to low body temperature.
The statements are listed below:
“It is suggested that pregnant bitches not be vaccinated. This suggestion is made as a result of our lack of information with regard to the possible side effects of the virus on the fetus and not from any results indicating that vaccine virus can damage the canine fetus.”
“The age of the dog is important not only because of persistence of colostral antibody, but also because the relative hypothermia that exists during the first week or two of life can cause a state of CMI unresponsiveness. Optimum body temperatures between 38 and 39° C. are very critical for T cell as well as macrophage function in the dog. Body temperatures of less than 37° C. are not uncommon in the puppy during the first week or so of life, and this lower body temperature is capable of suppressing the CMI system, although humoral immunity (antibody production) does not appear to be affected to the same extent as CMI. Vaccination during this early period (i.e., less than 2 weeks of age) with live attenuated vaccines is not recommended.